What is Reactive Dye? Properties and reaction of Reactive dye, Classification of Reactive Dyes
Content :
Reactive Dye
Structure & properties of Reactive Dyes
Reaction of Reactive dyes
Dye take up of Reactive dye
Classification of Reactive dyes
Reactive dyes
- For a long time, chemist had been seeking a
method of joining dye with cellulose through
covalent bond.
- At the end of 1940, chemists turned their
attension to the dyes containing cyanuric chloride
or triazinyl dyes
- It is possible to make chlorine atom
in cyanuric chloride to combine
with hydroxyl or amine group of
dye molecules
 |
| Reactive Dye |
An outstandingly important property of
cyanuric chloride is that if any chlorine atom
left unsubstituted, that can react to cellulose.
• The reaction of DCT with cellulose is shown
below
 |
| reaction of DCT with cellulose |
If hydrolysis occurs, that will decrease the
color yield and reactive dye converts into a
sort of direct dye which is very substantive to
the cellulose and also create problem in
washing off leading to poor wet fastness
Exploitation of the
dichlorotriazine reactive
system soon led to parallel
development of the much
less reactive
mono chlorotriazine dyes,
readily made by a
substitution reaction
between an aryl amine and
the dichlorotriazine
precursor.
 |
| Reaction between an aryl amine and the dichlorotriazine precursor. |
• More stable padding liquors
could be prepared using the
aminochlorotriazine types
Reaction of Reactive Dyes
Remazol (HOE) dyes, based on the 2-sulphatoethylsulphone
precursor of the vinysulphone reactive system or related species,
function by a nucleophilic addition mechanism rather than
substitution.
• Before this can occur, however, alkaline 1,2-elimination of the
precursor grouping is necessary to release the reactive
vinylsulphone system.
• In this system the carbon–carbon double bond is polarised by the
powerfully electron-attracting sulphone group.
• This polarisation confers a positive character on the terminal carbon
atom, favouring nucleophilic addition of either a cellulosate anion
or a hydroxide ion, again leading to either fixation or hydrolysis
respectively
Reactive dyes (Bifunctional)
• The appearance of two further interesting ranges of
bifunctional dyes that are capable of reacting with
cellulose via both mechanisms, nucleophilic substitution
and nucleophilic addition.
• In both systems one ring substituent in a
halogenotriazine dye carries a 2-sulphatoethylsulphone
grouping .
• The halogeno substituent can be either chlorine or the
more reactive fluorine.
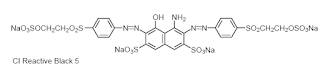
The four solubilising groups in the precursor form of CI Reactive
Black 5 confer high solubility but unusually low substantivity.
• It is a nearly symmetrical bis(sulphatoethylsulphone) structure
and as these precursor groups lose their ionic charge by 1,2-
elimination, the substantivity for cellulose is enhanced and the
bis(vinylsulphone) structure formed shows excellent fixation
efficiency under alkaline conditions.
Aminofluorotriazine sulphatoethylsulphone dyes
• Early in 1988 Ciba-Geigy launched the Cibacron C range of mixed
bifunctional dyes.
• They contain a new aliphatic vinylsulphone system and either a
monofluorotriazine bridging group or an arylvinylsulphone function.
• They are designed mainly for pad applications and appear to be
characterised by medium to low affinity, good build-up, easy wash-off and
high fixation.
• Their outstanding bath stability and high fixation make them especially
suitable for pad–batch dyeing.
• The manufacturing cost of these structures is believed to be relatively high
but the purchase cost to the dyer may be offset by enhanced costeffectiveness in use attributable to efficient fixation and easy wash-off,
possibly the best approach that time towards environmentally acceptable
reactive dyes.
Structure and properties of reactive dye
• The design of reactive dye structures almost always involves one or
more compromises between conflicting requirements.
• There is seldom an ‘ideal’ structure of a desired hue that embodies
all possible attractive features with regard to application and
fastness properties.
• The gain in aqueous solubility provided by an extra sulpho group
often has to be paid for by a decrease in affinity for cellulose.
• Enhancement of substantivity is beneficial for high exhaustion but
may impair migration or washing-off characteristics.
• High reactivity offers the possibility of rapid fixation but storage
stability may be adversely affected.
Reactive dyes uptake
• All conventional reactive dyes for cellulose, irrespective of
whether they react by nucleophilic addition, substitution,
or both mechanisms rely on the reactivity of the cellulosate
anion as the nucleophilic reagent and hence hydrolysis of
the dye by reaction with hydroxide ions from water will
always compete with the desired fixation reaction.
• Reaction between the dye and cellulose can occur only
when the dye has been absorbed into the cellulose phase.
Thus the kinetics of the dye–cellulose reaction are strongly
influenced by the rate of absorption of dye.
• The ratio of the rate constants for reaction of the dye with
the fibre and with water is a constant for a given dye over a
wide range of alkaline pH values.
Factors governing dye uptake
The efficiency of fixation is a function of:
1. The substantivity ratio, the relative concentrations of
dye absorbed into the substrate and remaining in the
dyebath;
2. The reactivity ratio, the ratio of rate constants for the
fixation reaction and hydrolysis;
3. The diffusion coefficient of the dye in the substrate;
4. The liquor ratio; and
5. The surface area of the substrate available for
absorption of dye.
Factors governing dye uptake
• The lower the linear density of the fibre, i.e. the
greater the surface area per unit weight, the more
efficient is the dyeing.
• The substantivity ratio is the most influential of the
factors governing fixation efficiency. Dyes of higher
substantivity diffuse more slowly than less substantive
dyes.
• Changes in dyebath conditions that increase
substantivity tend to decrease the diffusion coefficient.
• Lowering the liquor ratio favours increases in the rate
and efficiency of fixation.
• An increase of dyeing temperature lowers the
substantivity ratio and accelerates the rate of
hydrolysis of the dye; both of these effects
reduce the fixation efficiency.
• The rates of diffusion into and reaction with the
fibre are also accelerated, however, and these
factors both favour fixation of the dye.
• An increase in electrolyte concentration always
enhances substantivity without impairing
reactivity providing the dye remains completely
dissolved.
D
Classification of reactive dyes
Alkali-controllable reactive dyes:
• These dyes have optimal temperatures of fixation
between 40 and 60°C.
• They are characterized by relatively low exhaustion in
neutral salt solution before alkali is added.
• They have high reactivity and care in addition of alkali
is necessary to achieve level dyeing, preferably at a
controlled dosage rate.
• Typical examples of dyes belonging to this group have
dichlorotriazine, chlorodifluoropyrimidine, and
vinylsulphone reactive systems.
Salt-controllable reactive dyes
• Dyes in this group show optimal fixation at a
temperature between 80°C and the boil.
• Such dyes exhibit comparatively high exhaustion
at neutral pH, so it is important to add salt
carefully to ensure that dyeing is level.
• Electrolyte addition is often made portion wise or
preferably at a controlled rate of dosage.
• Dyes with these properties typically have lowreactivity systems such as trichloropyrimidine,
aminochlorotriazine or bis(aminochlorotriazine).
Temperature-controllable reactive dyes
• This group is represented by those dyes that react with
cellulose at temperatures above the boil in the absence of
alkali, although if desired they can be applied under the
same conditions as the salt-controllable group with alkaline
fixation at a temperature between 80°C and the boil.
• Dyes in this group have self-levelling characteristics so
there is no need to use auxiliary products to facilitate level
dyeing.
• Good results can be achieved by controlling the rate of
temperature rise. At present only the Kayacelon React (KYK)
range of bis (aminonicotinotriazine) dyes belong to this
group.





Post a Comment
Post a Comment